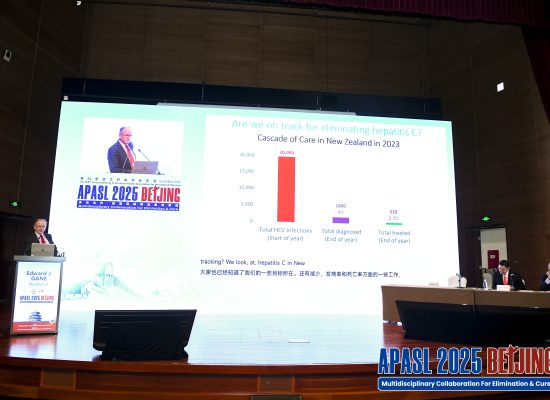
In medical research, especially in medical trials like those carried out by NZCR, it's important that people fully understand what a clinical trial involves, as well as the risks and benefits. So, before someone joins a clinical trial, NZCR must give potential participants all the details about the study.